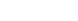Abstract
The generation, control and transfer of triplet excitons in molecular and hybrid systems is of great interest owing to their long lifetime and diffusion length in both solid-state and solution phase systems, and to their applications in light emission, optoelectronics, photon frequency conversion and photocatalysis. Molecular triplet excitons (bound electron–hole pairs) are ‘dark states’ because of the forbidden nature of the direct optical transition between the spin-zero ground state and the spin-one triplet levels. Hence, triplet dynamics are conventionally controlled through heavy-metal-based spin–orbit coupling or tuning of the singlet–triplet energy splitting via molecular design. Both these methods place constraints on the range of properties that can be modified and the molecular structures that can be used. Here we demonstrate that it is possible to control triplet dynamics by coupling organic molecules to lanthanide-doped inorganic insulating nanoparticles. This allows the classically forbidden transitions from the ground-state singlet to excited-state triplets to gain oscillator strength, enabling triplets to be directly generated on molecules via photon absorption. Photogenerated singlet excitons can be converted to triplet excitons on sub-10-picosecond timescales with unity efficiency by intersystem crossing. Triplet exciton states of the molecules can undergo energy transfer to the lanthanide ions with unity efficiency, which allows us to achieve luminescent harvesting of the dark triplet excitons. Furthermore, we demonstrate that the triplet excitons generated in the lanthanide nanoparticle–molecule hybrid systems by near-infrared photoexcitation can undergo efficient upconversion via a lanthanide–triplet excitation fusion process: this process enables endothermic upconversion and allows efficient upconversion from near-infrared to visible frequencies in the solid state. These results provide a new way to control triplet excitons, which is essential for many fields of optoelectronic and biomedical research.
Main
Figure 1a shows a schematic of the lanthanide (Ln)-doped nanoparticles (nanocrystals of NaLnF4) and the structures of some of the model molecules used in our study (rubrene and tetracene derivatives) along with their triplet energies. Unlike semiconductor quantum dots, these lanthanide-doped nanocrystals are insulators and their optoelectronic properties are governed solely by the lanthanide ions. We begin by preparing blended films of rubrene and NaGdF4 nanocrystals by drop-casting.
Fig. 1: Lanthanide-nanocrystal-coupled triplet excitation.
a, Schematic illustration of a lanthanide-doped nanocrystal (NaLnF4) and the organic molecules in our study. Note that ET refers to the triplet energy of the molecules. b, Comparison of the PDS spectra of films of NaGdF4–rubrene, NaLuF4–rubrene, NaYF4–rubrene with pristine rubrene and with NaGdF4 only. Only the system in which the lanthanide has unpaired spin (that is, the NaGdF4–rubrene films) shows a broadband absorption from 700 nm to 1,100 nm; the inset shows that this absorption matches the calculated direct transition from the ground singlet state (S0) to the lowest triplet state (T1). c, Comparison of the absorption spectra of 5-CT coupled with various lanthanide nanoparticles, including NaGdF4, NaErF4, NaLuF4 and NaYF4. Enhanced NIR absorption, related to the direct excitation of triplets, is only observed in the presence of lanthanide ions with unpaired spins (Gd3+ and Er3+) d, Comparison of absorption spectra of NaGdF4 nanocrystals coupled with tetracene derivatives (5-CT, CPT, CPPT). The enhanced NIR absorption decreases with increasing spacing between the lanthanide nanoparticle and the core of the molecule.
Fig. 2: Ultrafast intersystem crossing in organic molecules coupled to lanthanide-doped nanoparticles.
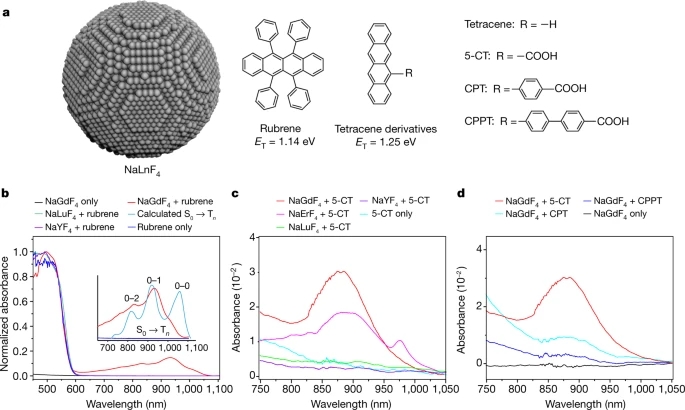
a, Schematic illustration of a NaYF4@NaLnF4 core–shell nanoparticle modified with CPPOA. b, Extracted kinetics showing the singlet (S1) decay and triplet (T1) growth of a solution containing pristine CPPOA molecules and of a solution of CPPOA-modified NaYF4@NaGdF4 nanoparticles. The singlet lifetime decreases from 12.9 ns in the pristine CPPOA to 82.3 ps in CPPOA-modified NaYF4@NaGdF4, indicating greatly enhanced intersystem crossing (ISC). Note that ΔT/T refers to the fractional differential transmission signal of the probe in transient absorption spectra. c, The interaction between the lanthanides and the molecules accelerates the ISC from the singlet to triplet exciton states of the molecule. d, Kinetics of triplet generation and decay in CPPOA molecules attached to different types of core–shell nanoparticles. The compositions of the core–shell nanoparticles are NaYF4@NaEuF4, NaYF4@NaGdF4, NaYF4@NaTbF4, NaYF4@NaYbF4, NaYF4@NaLuF4 and NaYF4@NaYF4. The key at top left gives the fitted values of τLn for the increasing part of each curve. For lanthanides with unpaired 4f electrons (Tb3+, Eu3+, Gd3+ and Yb3+) enhanced ISC is seen, whereas those with no unpaired spins (Y3+ and Lu3+) show no obvious enhancement in ISC. An enhanced triplet decay (τTb,decay = 7.66 ns and τEu,decay = 883 ps) of CPPOA on the nanoparticles containing Tb3+ or Eu3+ suggests triplet energy transfer to the lanthanide ions.
Fig. 3: Triplet energy transfer from molecules to nanoparticles.
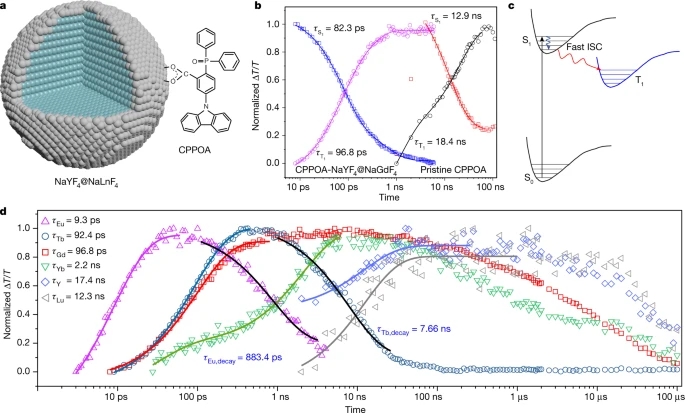
a, Simplified energy diagram showing triplet energy transfer (TET) from the molecular triplet state to lanthanide emitters (Ln3+) following a fast intersystem crossing (ISC) or singlet fission (SF) process. b, Photoluminescence spectra and corresponding luminescence photographs of colloidal solutions containing CPPOA-modified NaYF4@NaEuF4 nanoparticles (top) and CPPOA-modified NaYF4@NaTbF4 nanoparticles (bottom) under excitation at 365 nm. c, Photoluminescence spectra of NaGdF4:Yb–tetracene blend (green curve) and NaGdF4:Yb–rubrene blend (red curve) films excited at 405 nm. Luminescence arises from the transfer of triplet excitons generated via singlet fission to the 2F5/2 → 2F7/2 transition of Yb3+. The process is inefficient in rubrene owing to its triplet energy being lower than the 2F5/2 → 2F7/2 transition of Yb3+ (1.14 eV versus 1.25 eV).
Fig. 4: Lanthanide–triplet excitation fusion upconversion in nanoparticle–molecule blends.
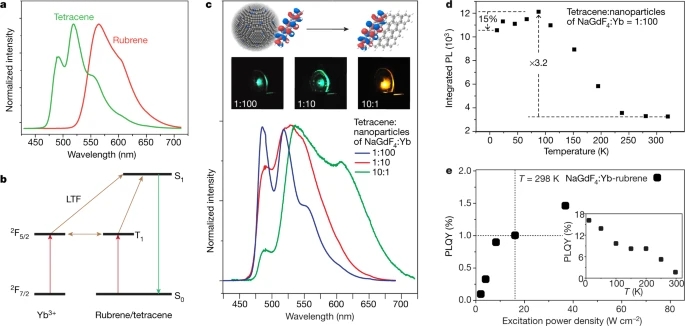
a, Photoluminescence spectra of NaGdF4:Yb–tetracene blend (50:1) and NaGdF4:Yb–rubrene blend (1:10) films excited at 980 nm (about 40 W cm−2), showing upconverted emission arising from the singlet state of the organics. b, Proposed lanthanide–triplet excitation fusion (LTF) upconversion process. c, Top, scheme represents the isolated molecule (left) and molecular conjugates (right) when tetracene couples with the NaGdF4:Yb nanoparticles. Note that grey and blue spheres in the nanoparticle refer to Gd3+ and Yb3+ ions, respectively. Bottom, upconversion spectra and corresponding emission photographs (middle) of NaGdF4:Yb–tetracene blend films with varying tetracene-to-nanocrystal ratios (1:100, 1:10 and 10:1). d, Plot of the temperature-dependent upconversion emission (integrated from 475 nm to 650 nm) of the NaGdF4:Yb–tetracene blend films (tetracene: NaGdF4:Yb = 1:100). Note that PL refers to photoluminescence. Dashed arrows suggests that upconversion emission showed a 3.2-fold increase as the temperature decreased from 300 K to 80 K and then a 15% decrease when the temperature was further reduced to 10 K. e, The internal photoluminescence quantum yield (PLQY) of the NaGdF4:Yb–rubrene blend measured as a function of excitation power density (excitation at 980 nm). Inset, temperature-dependent quantum yield of the same sample under excitation of 980 nm for a power density of 76 W cm−2.
DOI:10.1038/s41586-020-2932-2
https://www.nature.com/articles/s41586-020-2932-2