Abstract
Shape‐ and size‐controlled synthesis of micro/nanostructures is of fundamental importance in many applications of physics and chemistry. Wet chemical growth methods have achieved shape‐ and size‐controlled synthesis of colloidal nanocrystals of various compositions. Compared with wet chemical methods, electrochemical deposition (ECD) yields micro/nanostructures affixed to a substrate, but the resulting structures are poorly controlled. Herein, the controllable electrochemical fabrication of well‐defined silver‐oxide clathrate micro/nanostructures is realized by intentionally manipulating the previously neglected electrocarving process during electrodeposition growth (MEDEG). Most importantly, the dominance of the electrocarving and the electrodeposition growth process can be immediately manipulated by varying the deposition voltage and/or the composition of the electrolyte. Unique delta‐wing‐, arrowhead‐, and butterfly‐like silver‐oxide clathrate structures are created using the MEDEG method. MEDEG complements the capability of ECD for controllable synthesis of micro/nanostructures of various materials directly on a substrate. The study details the mechanisms that may enable MEDEG to become a competitive alternative to traditional wet chemical methods in the controllable synthesis of micro/nanostructures. This understanding of MEDEG should motivate applications in fields which demand well‐defined micro/nanostructures affixed to a substrate.
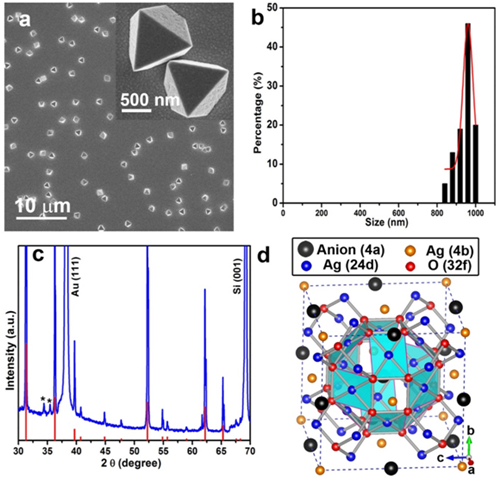
Figure1. Fabrication and characterization of Ag7O8NO3 pyramids. a) Scanning electron microscopy image. b) Size distribution obtained from measuring 200 pyramids. c) X‐ray diffraction. The red lines show the standard X‐ray diffraction pattern of Ag7O8NO3. The two asterisks mark weak peaks, and the Si and Au peaks come from the substrate. d) Crystal structure of Ag7O8NO3.
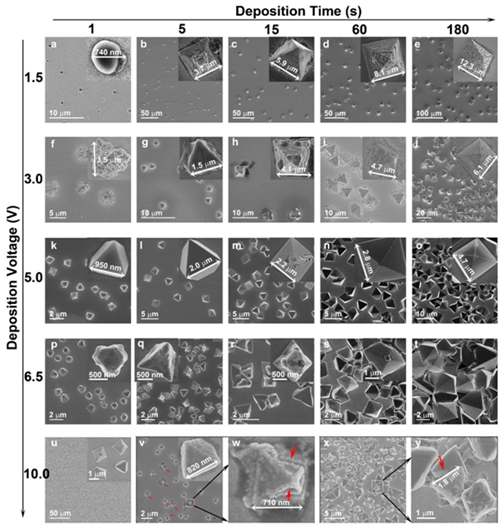
Figure2. Growth process of Ag7O8NO3 micro/nanostructures. Size and shape evolution of Ag7O8NO3micro/nanostructures at different deposition voltages for different times in electrolyte I. Inset: magnified image. Red arrows: etched area.
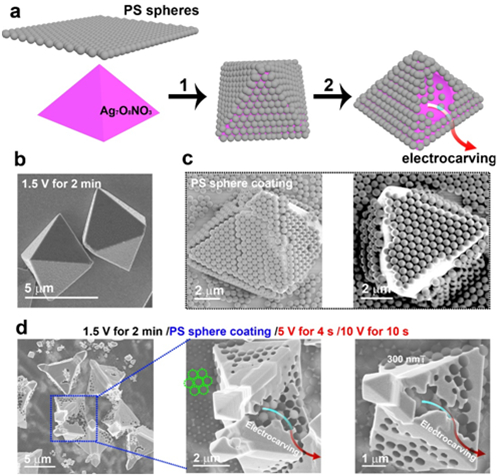
Figure 3. Consolidation of the existence of the electrocarving process. a) Ag7O8NO3 pyramids were covered by a monolayer of 500 nm PS beads (process 1), then electrodepostion was performed at 5 V for 4 s and 10 V for 10 s (process 2). b) The Ag7O8NO3 pyramids before PS sphere coating. c) The Ag7O8NO3 pyramids covered by a monolayer of PS spheres. d) Resuming electrodeposition. After electrodeposition, the monolayer PS sphere coating broke down upon electrocarving, with some PS spheres dropping inside the pyramids. The close packing of the PS spheres is indicated by the green dotted circles.
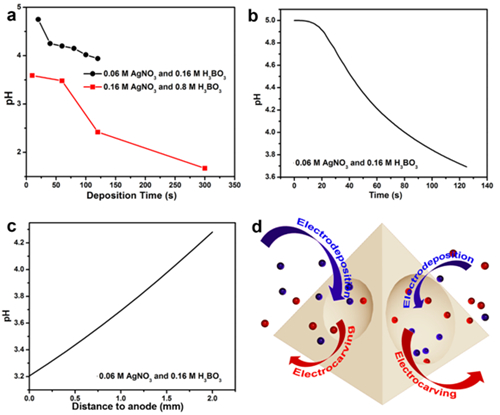
Figure 4. Experimentally measured and numerically simulated pH value change of the electrolyte close to the anode electrode surface during electrodeposition. a) pH value change during electrodeposition at 10 V for different deposition times in different electrolytes, measured by a pH meter placed about 1 mm away from the anode. b) Changes in the pH value at a position about 1 mm away from the anode during electrodeposition at 2.6 V simulated using COMSOL Multiphysics simulation software. c) The spatial profile of pH along the direction normal to the anode surface after 125 s deposition. d) Scheme of the formation mechanism of pyramids with concave surfaces by MEDEG.
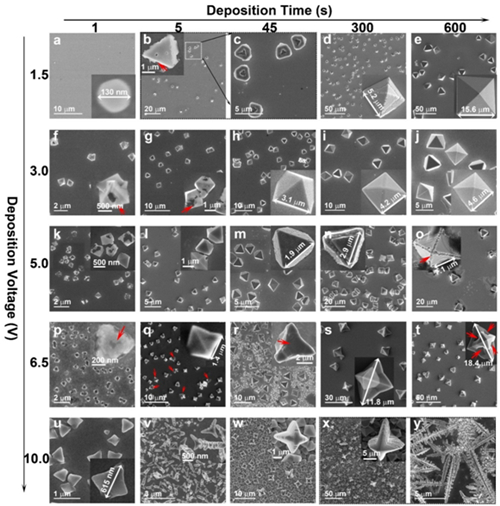
Figure 5. Ag7O8NO3 micro/nanostructures created by MEDEG. Size and shape evolution of Ag7O8NO3micro/nanostructures at different deposition voltages for different times in electrolyte II. Inset: magnified image. Red arrows: etched area.
In summary, electrocarving of the Ag7O8NO3 particles during electrodeposition growth was experimentally observed upon a decrease in the local pH. By manipulating the electrocarving and the electrodeposition growth process by varying the deposition voltage and/or the concentration of the boric acid in the electrolyte, Ag7O8NO3 particles with various unique microstructures have been fabricated. These electrodeposited Ag7O8NO3 structures can be used as templates in the deposition of other materials, and even leading to the formation of core–shell composite structures. This scenario of manipulating electrocarving during electrodeposition growth substantially strengthens the capability of ECD in size‐ and shape‐controlled synthesis of micro/nanostructures, which should ignite controllable synthesis of diverse materials using ECD. These materials with unique micro/nanostructures should find exciting applications in fields where there is a demand for well‐defined micro/nanostructures affixed to substrates.
Link:Electrocarving during Electrodeposition Growth. Adv. Mater. 2018, DOI: 10.1002/adma.201805686.
https://onlinelibrary.wiley.com/doi/10.1002/adma.201805686